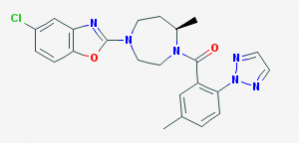
 Belsomra (suvorexant) was approved earlier this week by the FDA for use as needed to treat difficulty in falling and staying asleep.
Belsomra (suvorexant) was approved earlier this week by the FDA for use as needed to treat difficulty in falling and staying asleep.
The drug blocks receptors for orexin – also known as hypocretin – the master hypothalamic regulator of the sleep-wake cycle. There are, in fact, two forms of orexin, A and B; hence it is a dual orexin receptor antagonist (DORA).
Last July, Merck announced that the FDA had declined to approve suvorexant until the starting dose for most patients was 10 mg. The agency also said that proposed upper-limit doses of 30 mg for elderly patients and 40 mg for nonelderly patients were unsafe.
The FDA approved suvorexant at 4 different strengths — 5 mg, 10 mg, 15 mg, and 20 mg. The total dosage in 1 day should not exceed 20 mg.
Merck noted in its own news release today that the recommended dose is 10 mg for most patients, just as the FDA insisted.
In next-day driving tests that the FDA asked Merck to perform, both male and female participants who took the 20-mg dose proved to be impaired drivers. The FDA advises physicians to caution patients at this dosage level against next-day driving or other activities requiring full alertness. Patients taking lower doses also should know about the risk for impaired driving the day after because sensitivity to the drug varies from person to person.
